
Chemistry
Chapter 1: 1-6 Introduction to the study of chemistry

Homework
Homework Expectations for Chemistry Course
This homework page has some extra introductory material to help you get started.
Organizing your Work
The following list gives you a general idea of each task you need to complete for our meetings. You may want to copy it to another file or spreadsheet to help you keep track of your assignment completion.
- To prepare for the next honors course chat session:
- ____Check the homework page on the course content site for reading assignments, weblecture links, study activities, optional web reading
- ____Read the textbook sections assigned, making notes for review and listing questions to ask in chat
- ____Read the web lecture posted by the teacher, making notes for review and listing questions to ask in chat
- ____Complete the mastery exercises for the section.
- ____Complete any study activities, such as interactive lab simulations, listed on the homework page.
- ____Watch suggested videos, especially if you are having problems with particular concepts.
- ____Write your answer to the question assigned to you in the forum
- ____Study the lab assignment and make note of any questions you have on performing the lab; be sure to ask these in chat or email the instructor
- ____Attend chat and ask your questions, defend your forum essay, and participate in the discussion
- After completing a chapter in the text (check Moodle for due dates)
- ____Complete the mastery exercises and achieve 85% or better
- ____Take the textbook-based concept drill for review
- ____Take the Moodle Chapter Quiz: note these have limited availability dates and plan accordingly!
- ____Perform the assigned lab, analyze your data, and post your lab report by the due date
Getting to know the textbook
Take the time to become acquainted with the organization of the textbook, so that you can easily find things as we work through it.
- Table of Contents: The text has two different tables of contents. The first (preface p. iii) is a "brief" table listing the Parts, Chapters, Review Sections, and Appendices. Immediately following this page is the detailed table of contents, which lists sections, subsections, chapter goals, key equations, and study questions for each chapter and review section. This is a good place to come when starting a new chapter or unit, so that you can get an idea of the topics that will be covered, and how they fit together.
- What are the topics of chemistry?
- What is the order in which they are presented?
- Prefatory materials: The preface covers the textbook's pedagogical goals and helps, and gives you some of the authors' rationale in putting it together a particular way.
- Chapter heading: The opening section for each chapter includes a brief description of some chemical concept or problem that sets the theme for the chapter. Pay attention to the chapter goals and outline.
- Chapter sections: Each major topic is marked off by a numbered chapter section. These often include numbered exercises (with answers in the back of the book -- AITBOTBs), and numbered formulae. Pay attention to these!
- Tables: The chapter may include summaries of quantitative or related data. Often these are sources of information for homework problems, so be ready to come back and check their contents when doing homework problems.
- Graphs, images, and diagrams: The preface for this edition explicitly states that information has been moved into the diagrams from the captions, so you need to spend time examining any pictures carefully.
- Chapter review helps: The end of each chapter restates the goals of the chapter, and points you at the sections where the goal was covered, as well as the study questions that test your comprehension. Key equations may be listed as well. You will be assigned homework problems from the Study Questions at the end of each chapter.
- Appendices: At the end of the text are a number of appendices. You may want to pay special attention to some of these! You want to make a note to check this list if your homework assignment seems to assume that you know some particular value. You may just need to look it up in one of these appendices.
- A: Mathematical summary of logarithms and the quadratic equation.
- B: Physical concepts for review
- C and D: Conversion factors and physical constants
- E: Naming conventions
- F-M: Specific measured quantities used in calculations for different reactions
- N: Answers to the in-text exercises, some of the study problems, and the case study questions that open each chapter. Remember that the correct numerical answer only counts for 20% of your homework problem score: the other 80% depends on how well you explain your procedure and thought processes in getting that number!
Chapter 1 Homework
[Most homework pages will start with your reading assignment, like this:]
- Reading Preparation
- WebLecture
- Study Activity
- Preparation work for chat
- Online Quiz
- Lab Instructions
Reading Preparation
Textbook assignment: Read Kotz and Triechel, Chemistry and Chemical Reactivity, Prefaces and Chapter 1: Sections 1-8.
General Preface
Review the General Preface and About the Authors (xix-iiiiv). Pay particular attention to how the text is laid out, the purpose of chapter goals and focus pages, and the recommended use of example problems, section exercises and study questions.
Chapter 1 MATTER AND MEASUREMENT: Sections 1-8
The introductory chapter of our text is typical of most textbooks: it was written to entice you with some of the more interesting (to the authors of the text at least) aspects of chemistry, and in response to some popular sense of how chemistry is useful. For their own purposes, the authors are simply happy to have knowledge that gives them some sense of control over the universe — and for most pure scientists, this is enough. Even if they can't do anything about a situation, at least they know why it is happening, and why this event is different from other events.
For practical purposes, the authors offer the currently popular (and real) circumstance that as future voters, you will determine how research dollars are spent and how laws are enforced, particularly environmental and public health laws, based on research done by chemists. If you cannot properly evaluate and understand the results of chemical research at some level, you will not be able to make informed decisions about issues that rest on the professional chemists' understanding of the way the universe works. At the very least, as a consumer of chemicals (every material object you buy is made of them, after all), you should have some sense of what you are spending your money to acquire.
Christians (and Jews and Moslems and anyone else who believes in a created universe) have the additional responsibility of appreciating the nature of the universe because it may tell us something about the Creator. We accept the idea that the natural world is real and than it follows certain ordained laws, that its behavior is regular and therefore predictable. The more we understand about the complexity of the relationships of particulate matter, the more we can wonder at the Mind that thought all of this up.
The study of chemistry links us to a long line of philosophers and theologians and scientists who were fascinated by the forms of matter and the ways it changes from one form to another. Welcome to Chemistry....and to the company of Thales of Miletus, Aristotle, Paracelsus, John Dalton, and Linus Pauling, just to name a few.
As you read this material, keep in mind the following questions:
- How does the study of chemistry differ from the study of other sciences?
- What are some of the practical benefits and risks of chemical research?
- What is scientific methodology, and how is the experimental method modified by the realities of the society in which it takes place?
- What are some of the moral and ethical issues that face chemists in particular?
Details matter
Chapter 1 also introduces us to (and sometimes assumes that we know something about) the most basic concepts and the most common experiences we share about matter. While some of these ideas are thousands of years old, others date back only a century or two. Humans have long been aware of the three common states of matter, solid, liquid, and gas, because one of the chemicals fundamental to all life —water — can assume all of them within most normal weather temperature ranges. Humans have also been in pursuit of pure substances —things that cannot be "split" into component substances — for centuries as well, and early identified certain metals like gold and silver as "pure". The breakdown of substances into atoms and molecules is much more recent, and the realization that the molecules move around according to their heat content (as measured by their temperature) is more recent still.
As you read these sections, pay attention to the definitions of the basic terms:
- What is the difference between a substance and a mixture?
- What is the difference between the terms "element" and "atom" (people sometimes use them interchangeably)?
- When is a molecule a compound and when is it not a compound?
- What is the difference between a basic quantity like mass or volume, and a calculated quantity like density?
- What does temperature really measure?
- What are the physical properties of a substance, and how can they be measured?
- What is the difference between physical and chemical change?
- What is the "kinetic-molecular theory" of matter, and how does it explain the states of matter (solid, liquid, gas)?
- What do we mean by macroscopic and particulate levels of matter? How does our explanation of the behavior of matter change when we change "levels"?
- Why are standard units of measurement important? How (and when!) do you convert from one type of unit to another?
When you get to an in-text example, stop and work it out. The answers are in the back of the book. These "checkpoints" will help you identify whether or not you really understand the material just presented.
Study Notes
- 1.1 Chemistry and its Methods. The authors offer several reasons to study chemistry:
- it is interesting in and of itself
- it is the basis or contributor to other sciences, including biology, geology, and astronomy
- it has practical applications in the creation and production agricultural supplements (fertilizers), materials for electronics, plastics, and ceramics, not to mention pharmaceuticals, and is fundamental for waste management.
- as a discipline, it uses methods of observation, analysis,and synthesis that applicable to other sciences
- public policy and health issues required an informed population
Chemical methods involve the application of qualitative and quantitative observation methods to form and test hypothesis, to discover natural laws governing the relationships between physical characteristics, and to eventually construct theories that help explain many related phenomena as the result of consistently acting forces of nature.
To ensure that these methods are properly applied, and recognizing that humans may act with less than pure motives and perfect integrity, modern scientific communities have a system of checks and balances that require independent groups to check each others work before the scientific community as a whole accepts the claims of any one scientist or organization.
- 1.2 Sustainability and Green Chemistry. This is a new section, added by the real (and responsible) need to consider how man-made chemicals change our environment. While this is often expressed as a purely practical and secular concern, it does reflect the fundamental Christian, Jewish, and Islamic concept that man should be a steward, not owner or exploiter, of the universe God created.
- 1.3 Classifying Matter. In order to talk the various characteristics of matter, we often identify matter by its macroscopic state (solid, liquid, gas), which results from the energy and motion of its component particles (atoms, ions, or molecules). Atoms combine to form molecules that exhibit different types of combinations as substances, mixtures, and solutions.
- 1.4 Elements and Atoms. Atoms are the fundamental units of chemistry, distinguished into types (elements and isotopes) by the number and kind of subatomic particles they contain.
- 1.5 Compounds and Molecules. Compounds are combinations of different atoms; molecules are combinations of atoms that involve chemical bonds, such that the elements cannot be separated by purely physical means, as mixtures can.
- 1.6 Physical Properties. Physical properties are the macroscopic observable properties such as density, boiling and melting points, malleability, and ductility. The set of these properties unique to a given substance and can be used to identify the substance. Intensive properties depend only on the type of substance; extensive properties depend on the amount of substance present as well.
- 1.7 Physical and Chemical Changes. Physical changes do not change the identity of a substance: water is water whether it is in the form of ice, or liquid water, or steam. Chemical changes cause molecules to recombine, so that the reacting substances reform into new product substances with different physical characteristics.
- 1.8 Energy: Some basic concepts. While chemistry focuses on the characteristics of matter, this matter is still bound and controlled by forces that do work and manipulate energy. We will be considering how potential energy (due to position in force fields) and kinetic energy (due to motion) affect different kinds of matter, and using the principle of the conservation of energy to predict the effects of energy-using and energy-producing chemical reactions.
Important Formulae
| Concept | Relationship | Formula | Symbols | Typical Units |
|---|---|---|---|---|
| Volume of cube | length * width * height | V = l * w * h | V: volume l: length w: width h: height |
cm3, m3 |
| Volume of sphere | volume to radius | V = 4πr3 | V: volume l: length w: width h: height |
cm3, m3 |
| Density | mass/volume | ρ: density m: mass V: volume |
g/cm3, kg/m3 |
Web Lecture
Read the following weblecture before chat: Introduction to the study of Chemistry
Take notes on any questions you have, and be prepared to discuss the lecture in chat.
Study Activity
Videos for Chapter 1
Our textbook publisher has a video website that contains short 3-5 minute videos on selected topics. As we go through chapters, I'll point out videos you may want to view, especially if you are having problems visualizing the material simply by reading it. You should bookmark the Thinkwell link to the index page here...and after study points on homework pages, I'll suggest which videos you may want to view on specific topics.
For this part of chapter 1, you my want to review the videos listed below. You'll have to experiment and determine whether you find it more useful to watch the videos before or after reading the text.
Go to Thinkwell Video Lessons and review the following six videos:
- An Introduction to Chemistry and the Scientific Method
- An Introduction to Chemistry
- The Scientific Method
- Properties of Matter
- States of Matter
- A Word about Laboratory Safety
- CIA Demonstration: Differences in Density Due to Temperature
- Properties of Matter
Chat Preparation Activities
- Essay question: The Moodle forum for the session will assign a specific study question for you to prepare for chat. You need to read this question and post your answer before chat starts for this session.
- Mastery Exercise: The Moodle Mastery exercise for the chapter will contain sections related to our chat topic. Try to complete these before the chat starts, so that you can ask questions.
Chapter Quiz
- Required: Complete the Mastery exercise with a passing score of 85% or better.
- Go to the Moodle and take the quiz for this chapter.
Lab Work
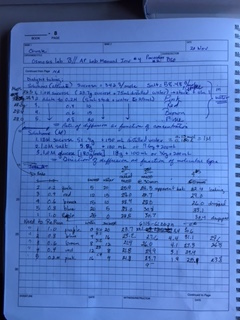
Please read the introductory materials -- preface and table of contents -- and Chapter 1, pp. 1-7, of your lab work book: Illustrated Guide to Home Chemistry Experiments: All Lab, No Lecture. You may also find this set of Instructions for Using Your Laboratory Notebook from MIT useful. As you can see, you lab notebook will probably not look very neat, but it should be possible to read anything you wrote down, even if you decide later that it was wrong.
Online Resources
The "hybrid" edition of textbook uses the OWLv2 site which is included with the purchase of a new copy of the text. I do not recommend that you purchase this version of the text, but if you did and your text did not come with end-of-chapter exercises, please let me know. I do not require that you purchase access to OWLv2 for this course.
OWLv2 does give you access to MindTap, a dynamic and interactive set of exercises and presentations that supplement the text materials. If you did purchase access, you should use these often to observe chemical reactions and processes and supplement your learning: you might as well get what you paid for! If you did not purchase the OWLv2 access, be sure to use the ThinkWell video resources (which are free) referenced in the blue box on homework pages to provide similar observing opportunities.
© 2005 - 2024 This course is offered through Scholars Online, a non-profit organization supporting classical Christian education through online courses. Permission to copy course content (lessons and labs) for personal study is granted to students currently or formerly enrolled in the course through Scholars Online. Reproduction for any other purpose, without the express written consent of the author, is prohibited.