Natural Science - Year II
Unit 38: Chemical Bonds and Reactions

Science Web Assignment for Unit 38
| This Unit's | Homework Page | History Lecture | Science Lecture | Lab | Parents' Notes |
Science Lecture for Unit 38: Chemical Reactions
For Class
- Topic area: Chemistry: Molecules and Bonds
- Terms and concepts to know: Association, dissociation, activation energy
- See historical period(s): 17th century - 19th century
Outline/Summary
Reactions
Consider again our water molecule. Dalton's laws of proportion and constant composition require that we have whole-number ratios of atoms, but this allows the same kinds of atoms to combine in different ways. By breaking the bonds that hold atoms together in one kind of molecule and rearranging them, we can get new substances. For example, we can break a water molecule (H2O) into two pieces, an hydroxide ion that contains one oxygen, one hydrogen, and the electron from the second hydrogen, simply by running current through the water. This gives the OH particle an excess negative charge, so we call it an ion, and write its formula as OH-. The second hydrogen is also an ion, missing an electron, so it has a net positive charge, and we write its formula as H+.
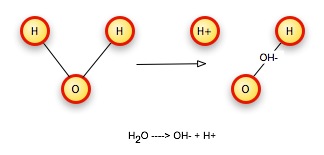
Balancing Equations: Conservation of matter
Dalton's rule that matter must be conserved means that we cannot lose or gain any atoms during the rearrangement process. So if we start with two hydrogen atoms and one oxygen atom, we have to end up with two hydrogen atoms and one oxygen atom.
A corollary to conservation of matter is the idea that charge is also conserved. We start with an electrically neutral water molecule, so the starting charge is zero. We wind up with two particles, one with a +1 charge and the other with a -1 charge, so the total charge +1 + -1 = 0 is again zero. Charge has been conserved.
Molecules can form or dissociate (as in the reaction above); they can also collide and recombine. We can start with two water molecules, and under the right conditions, a collision will split off a hydrogen from each water molecule, allowing the oxygens to bond directly to each other, and releasing the hydrogens to form a gas molecule.
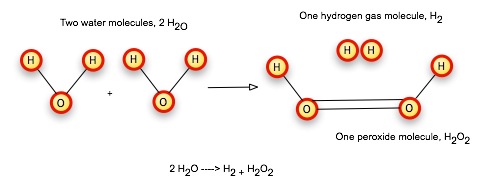
As you can see, we started with 4 hydrogens and 2 oxygens, and we end up with 4 hydrogens and 2 oxygens. Matter is conserved during each moment of the reaction.
Read the ChemTeam's Webpage on The Meaning of a Chemical Equation. To learn how you can balance chemical equations yourself, read Balancing Chemical Equations.
[2 web pages]
- What are the reactants of a chemical reaction?
- What are the products?
- What are the coefficients?
- What law of chemistry assures us that balancing chemical reactions is possible?
- What can you change when balancing a chemical reaction: the coefficient for a whole molecule or atom, the subscript (number of atoms in this molecule), or a coefficient within a molecule?
- Test yourself with the practice problems at the bottom of each page.
Activation Energy
Our current kinetic theory of molecular motion explains a reaction as a collision between two molecules. If the collision is hard enough (the molecules are moving quickly and hit at the right angle), the molecules will break apart and can then reform in a different combination. A weak collision means no breakup occurs, so there has to be a minimal amount of energy, called the activation energy, available to propel one molecule into another.
In many cases, we just need to add heat energy to a system of molecules to start a reaction. The heat energy causes the particles to speed up, collide, and break apart. Without this initial activation energy, no reaction can occur.
Now, every bond between two atoms is a kind of energy storage case. When we break the bond, we release energy. When we form the bond, we have to push molecules together and this takes work; the work energy is stored in the forming bond. Consider the following reaction:
CH4 + 2 O2 → CO2 + 2 H2O
We can explain this reaction this way: a molecule of methane (the CH4 molecule) combines with two molecules of oxygen gas (each of which has 2 atoms of oxygen in it) to form a molecule of carbon dioxide and two molecules of water. The number 2 in front of the reacting oxygen gas molecule (O2) means that we have 2 of the two-atom molecules, or 4 oxygens altogether, to balance the results, where there are 2 oxygens in the single molecule of carbon dioxide, and one in each of the resulting molecules of water.
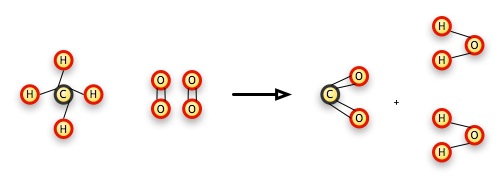
This is a combustion reaction, where we "burn" a substance in oxygen. With all hydrocarbons, or molecules made up of just hydrogen and carbon, the result is always carbon dioxide and water. The C — H bonds break and both the carbon atoms and hydrogen atoms form separate, new bonds with oxygen. In this particular case, the C — H bonds have more energy than the resulting C — O and H — O bonds. This energy has to go some place (just like matter, energy can't be created or destroyed), so it goes into the universe as heat, raising temperatures, causing sound, radiating as light, and some of it may go to cause other methane and oxygen atoms to speed up and collide, creating a second reaction that releases more energy.
When we have a system where the reacting molecules have more energy than the product molecules, the reaction becomes self-perpetuating. We say that it is a spontaneous reaction: we only need to put enough activation energy into the system of molecules to get the first reaction to occur. That reaction will release enough energy to cause another molecular interaction, and so on, until all the available reactants have been converted to products (or we've run out of one of them). Sometimes we say this reaction is exothermic because it gives off heat, or exergonic because it gives off some form of energy even it if isn't always heat.
Suppose now, we run the reaction the other way:
CO2 + 2 H2O → CH4 + 2 O2
Now we have a situation where the reactants have less energy in their bonds than the products. We get enough activation energy to cause the first reaction — but there is no energy left over to cause the second reaction. We have to put more energy into the system, and keep putting energy into the system, for the reaction to continue with all the available molecules of water or carbon dioxide. This kind of a reaction is endergonic, we have to put energy (ergons) into the system. Endergonic (or endothermic, if we talk specifically about energy in the form of heat) reactions are not spontaneous. This is probably a good thing in the case of the formation of hydrocarbons, or the reaction would run until all the carbon dioxide and water in our atmosphere was used up!
Types of Reactions
There are several major types of reactions.
| Type | General Formula | Example |
| Synthesis | A + B → AB | Na+ + Cl- → NaCl |
| Dissociation | AB → A + B | H2O → H+ + OH- |
| Recombination | AB + CD → AD + BC | CH4 + 2 O2 → CO2 + 2 H2O |
Synthesis reactions are necessary to build up complex molecules from simple components. All organic processes depend on synthesis reactions: proteins, complex carbohydrates and fats, and even DNA are build by a process called dehydration synthesis, which pulls out hydrogen and hydroxide ions from simple molecules so that they can be combined together to form long chains of molecules. Dissociation reactions are very important in acid-base reactions, since the first step is always for the hydrogen ion H+ to dissociate from its acid molecule. And as we have seen, combustion reactions require the recombination of atoms from initial reactant molecules into completely different molecules to release energy that we can use for work.
Precipitation Reactions
Many reactions occur in water solutions. We call these aqueous reactions. Salt dissolving in water is a common dissociation reaction that we depend on in cooking. Acids dissolving in water are also important reactions that must occur in solutions.
In some cases, if we dissolve two different chemicals in water, the dissolved ions of one compound may combine with the dissolved ions of another compound to form a solid, or precipitate. Powdery substances will float to the bottom of the reaction beaker or test tube. We say that these substances are not soluble in water. We can use the solubility reactions to isolate some molecules or elements, or to form new molecules easily.
Study/Discussion Questions
- How does the conservation of mass help us balance reaction equations?
- How does a reaction's need for or release of energy make it more or less likely to occur?
Further Study On your Own (Optional)
- Learn more about basic chemistry principles at the Basic Chemistry personal tutorial site. These lessons focus on organic chemistry, so you can see how chemistry concepts underly our current understanding of biology.
© 2005 - 2024 This course is offered through Scholars Online, a non-profit organization supporting classical Christian education through online courses. Permission to copy course content (lessons and labs) for personal study is granted to students currently or formerly enrolled in the course through Scholars Online. Reproduction for any other purpose, without the express written consent of the author, is prohibited.